- Be How Many Valence Electrons
- How To Determine Valence Electron
- Valence Electrons
- How To Determine Number Of Valence Electron
VALENCE ELECTRONS A VIEW FROM THE TEXAS EDUCATION AGENCY IPC 7D Oxidation Numbers When atoms gain or lose electrons they become either negative or positive When atoms lose electrons, they are positive When atoms gain electrons, they are negative Oxidation Numbers Group 1: 1+ Group 2: 2+ Group 13: 3+ Group 14: 4+/4- Group 15: 3- Group 16: 2- Group 17: 1- Group 18: 0 Oxidation Numbers When atoms. Valence electron definition is - a single electron or one of two or more electrons in the outer shell of an atom that is responsible for the chemical properties of the atom.
Learning Objectives
- Define valence electron.
- Be able to indicate valence electrons when given the electron configuration for an atom.
What makes a particular element very reactive and another element non-reactive?
Clicking on an atom in the structures below will add a lone pair of electrons. Clicking on a bond will add a pair of electrons to the bond (making a single bond a double bond). Arrange electrons around the atoms in each structure so each atom has an octet. The number of valence electrons for each molecule or ion is shown beneath the structure. Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms. Valence electrons are generally the electrons that are farthest from the nucleus. As a result, they may be attracted as much or more by the nucleus of another atom than they are by their own nucleus. Valence Electrons and Ions Worksheet Element Name Metal/nonmetal/m etalloid # Valence Electrons # Electrons to gain # Electrons to lose Ion Formed/ name Li Lithium metal 1 none 1 Li+/cation N nitrogen nonmetal 5 3 none N 3-/anion / / / / / / / Br bromine nonmetal 7 1 2 Br-/anion Xe xenon nonmetal 8 none none / Ca calcium metal 2 6 2 Ca 2.
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path a specific element will take depends on where the electrons are in the atom and how many there are.
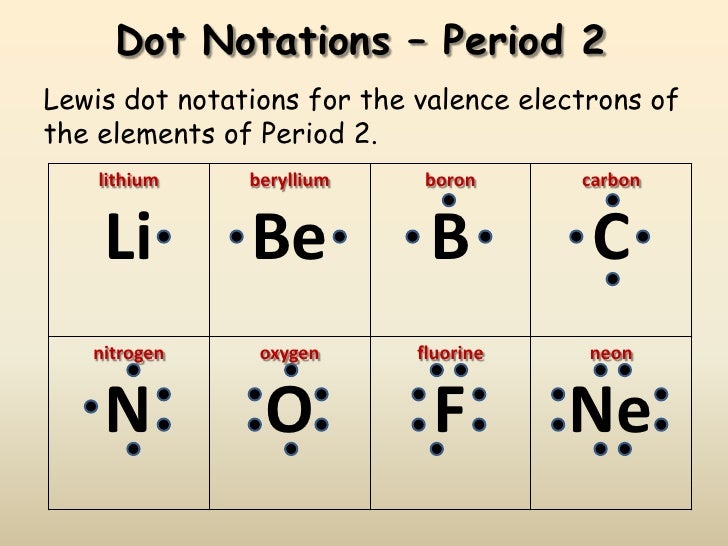
| Element Name | Symbol | Atomic Number | Electron Configuration |
| Lithium | Li | 3 | 1s22s1 |
| Beryllium | Be | 4 | 1s22s2 |
| Boron | B | 5 | 1s22s22p1 |
| Carbon | C | 6 | 1s22s22p2 |
| Nitrogen | N | 7 | 1s22s22p3 |
| Oxygen | O | 8 | 1s22s22p4 |
| Fluorine | F | 9 | 1s22s22p5 |
| Neon | Ne | 10 | 1s22s22p6 |
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
Summary
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.
Be How Many Valence Electrons
Practice
Use the link below to answer questions about valence electrons:
Review
- Define valence electron.
- Define inner shell electron.
- How many valence electrons are there in fluorine?
- What are the 2s electrons in nitrogen?
- How many inner shell electrons are there in beryllium?
How To Determine Valence Electron
Glossary
- inner-shell electrons: Those electrons that are not in the outer shell and are not involved in the reactivity of the element.
- valence electrons: The electrons in the highest occupied principal energy level of an atom.
Valence Electrons
References
How To Determine Number Of Valence Electron
- User:Chemicalinterest/Wikipedia. http://commons.wikimedia.org/wiki/File:Cobalt_carbonate.JPG.
